Pharmacogenetic Testing
Overview:
Pharmacogenetic testing (also referred to as pharmacogenomic testing or medication response test) is being used in the field of psychiatry to help in the decision-making process of selecting medication treatments. Results from two recent studies indicated that “personalized medicine” did significantly improve response and remission rates for difficult-to-treat depression patients over the standard-of-care. The studies also showed that the patients taking incongruent medications* at baseline (start of the studies) had their side effect significantly reduced when they were switched to congruent medications.
Science Behind the Testing:
Genes are expressed into RNA and transcribed into proteins that interact with psychotropic drugs. The cytochrome P450 (CYP) enzyme system is a protein superfamily involved in the synthesis and metabolism of drugs, toxins, and normal cellular components. The growing number of genetic testing companies analyze the genes that code for specific CYP enzymes to determine a patient’s ability to tolerate or respond to medications cleared by the P450 system. However, numerous environmental and other factors also influence the extent to which particular genes interact. Genetic testing does not assess the combined, interactive effects of genetic, epigenetic, and environmental factors.
Clinical Use:
The genetic testing is not designed to guide doctors to the perfect medications but mainly helps them move away from drugs that have a high potential to cause side effects. The ideal patient to have the pharmacogenomic testing would be an individual who has tried and failed at least 2 different medications of different classes (based on the drug’s mechanism of action). Pharmacogenetic testing does not have clinical utility when used on stable patients.
Recommendations from Core Revitalizing Center:
- Providers should use pharmacogenetic testing as one of the numerous tools in the clinical decision-making process when providing personalized medicine.
- Pharmacogenetic testing is appropriate in patients who have failed one or more medications.
- Most Medicaid and Medicare plans cover the pharmacogenomic testing at 100%. Private insurances may or may not cover the testing; therefore the patient should contact their insurance company BEFORE the provider takes the sample.
- CRC uses the OneOme RightMed© comprehensive test (co-developed with Mayo Clinic). I have found the reports from the OneOme testing to be superior to that of Genesight, Millenium Health and other similar products. CRC does not receive any compensation from the manufacturers of the testing.
- If you have had pharmacogenetic testing or plan on completing the DNA swab, keep a copy of the results to bring to your medical providers.
OneOme Sample Reports:
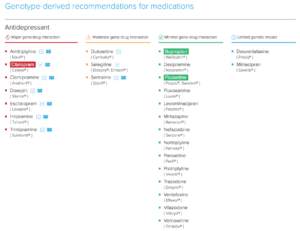
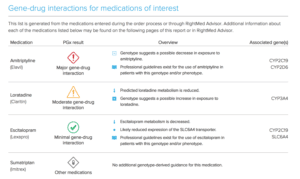

*Medications found to be in the “use with increased caution and more frequent monitoring” category.
Greden JF, Parikh SV, Rothschild AJ, Thase ME, Dunlop BW, DeBattista C, Conway CR, Forester BP, Mondimore FM, Shelton RC, Macaluso M, Li J, Brown K, Gilbert A, Burns L, Jablonski MR, Dechairo B. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. 2019 Apr;111:59-67. doi: 10.1016/j.jpsychires.2019.01.003. Epub 2019 Jan 4. PubMed PMID: 30677646.
Greden JF, Rosthschild AJ, Thase M, Dunlop BW, DeBattista DC, Conway CR, Forester BP, Mondimore FM, Shelton RC, Li J, Gilbert A, Burns L, Jablonski M, Dechairo B, Parikh S. 49 Combinatorial Pharmacogenomics to Guide Treatment Selection for Major Depressive Disorder: A Large, Blinded, Randomized Controlled Trial. CNS Spectr. 2019 Feb;24(1):202-203. doi: 10.1017/S1092852919000415. PubMed PMID: 30859976.


Recent Comments